Atrial flutter is often perceived as a straightforward arrhythmia, especially when it involves the cavotricuspid isthmus (CTI). However, atypical atrial flutter, particularly in the presence of atrial scar, presents a far more complex diagnostic and therapeutic challenge. Accurate identification of the re-entrant circuit is essential to ensure durable procedural success.
This case highlights the role of high-density electroanatomical mapping, voltage threshold adjustment, and propagation analysis in identifying and treating a scar-related mitral annular–anterior wall atypical atrial flutter.
Understanding Atypical Atrial Flutter
Unlike typical CTI-dependent flutter, atypical atrial flutter:
-
Arises from non-CTI circuits
-
Often involves the left atrium
-
Frequently depends on prior ablation scars, fibrosis, or structural remodeling
-
May demonstrate variable cycle lengths and activation patterns
These features make surface ECG interpretation insufficient, necessitating a comprehensive electrophysiology (EP) study.
Patient Presentation
A 52-year-old female presented with:
-
Recurrent palpitations
-
Episodes of pre-syncope
Initial Evaluation
-
ECG: Ongoing atrial flutter
-
Clinical decision: Proceed with electrophysiology study and radiofrequency ablation (RFA) due to persistent symptoms
Baseline Electrophysiological Findings
-
Sustained atrial flutter with a cycle length of ~230 ms
-
Coronary sinus activation suggested left atrial involvement
-
Findings were inconsistent with CTI-dependent flutter
These features raised strong suspicion for atypical left atrial flutter.
High-Density Left Atrial Mapping
Why High-Density Mapping Matters
Standard mapping may fail to:
-
Detect slow conduction channels
-
Identify protected isthmuses within scar tissue
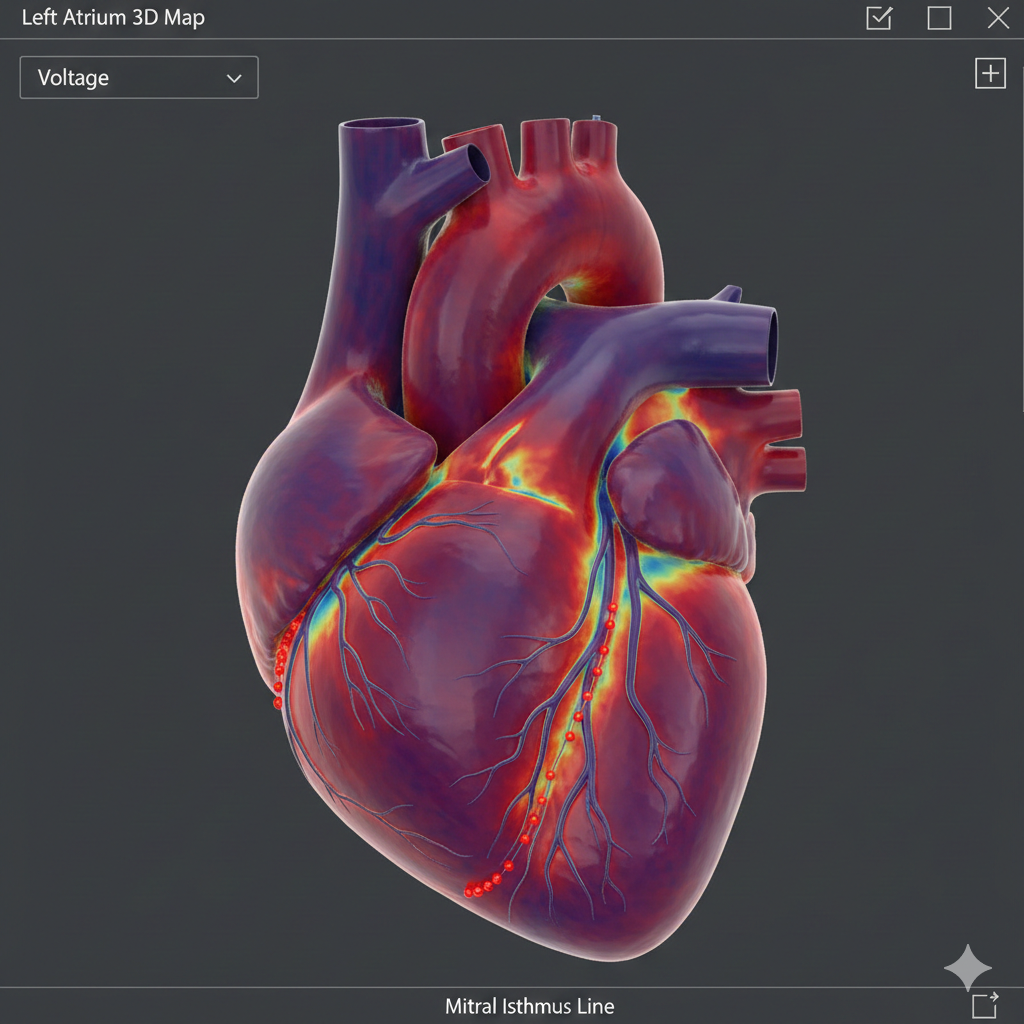
Using HD Grid mapping, a detailed anatomical and electrical reconstruction of the left atrium was achieved.
Key Observations
-
Re-entrant activation pattern identified
-
Slow conduction zone localized to the anterior left atrial wall
-
Evidence of scar-related conduction heterogeneity
Induced Tachycardia and Circuit Evolution
Following initial ablation attempts:
-
Tachycardia terminated but was reinducible
-
Reinitiated flutter showed:
-
Prolonged cycle length (~250 ms)
-
Altered activation sequence
-
This indicated partial circuit modification, not complete elimination.
Entrainment and Circuit Confirmation
Entrainment mapping demonstrated:
-
CTI: OUT of the circuit
-
CS 5–6: IN the circuit
These findings confirmed a non-CTI–dependent, left atrial macro-reentrant flutter.
The Challenge of Scar-Related Flutter
Repeat activation mapping initially failed due to:
-
Extensive low-voltage scar
-
Electrical silence masking true conduction pathways
Solution: Voltage Threshold Adjustment
By lowering the low-voltage cutoff and refining fractionation parameters:
-
Hidden conducting channels within scar became visible
-
The critical pathway sustaining flutter was unmasked
Propagation Mapping: Defining the Mechanism
Propagation maps clearly demonstrated:
-
A macro-reentrant circuit involving:
-
The mitral annulus
-
The anterior left atrial wall
-
-
A critical connecting isthmus bridging these regions
This confirmed a mitral annular–anterior wall atypical atrial flutter.
Targeted Ablation Strategy
Ablation Site
-
The connecting slow-conduction channel
-
Characterized by:
-
Fractionated mid-diastolic electrograms
-
Consistent participation throughout the tachycardia cycle
-
Procedural Outcome
-
Progressive slowing of tachycardia
-
Termination of flutter during RF energy delivery
-
No alternate arrhythmia induced
Termination during ablation strongly validated the critical isthmus.
Post-Ablation Results
Rhythm Outcome
-
Restoration of stable sinus rhythm
-
No inducible atrial flutter
-
Sustained rhythm stability during post-procedural monitoring
Post-RFA ECG
-
Normal sinus rhythm
-
Absence of flutter waves or tachyarrhythmia
Key Clinical Takeaways
-
Atypical atrial flutter often hides within scar-related conduction channels
-
Voltage adjustment is essential when standard mapping is inconclusive
-
Propagation mapping is invaluable in defining true circuit anatomy
-
Termination during ablation confirms mechanistically targeted therapy
Conclusion
This case underscores that successful treatment of atypical atrial flutter requires more than anatomical assumptions. High-density mapping, intelligent voltage adjustment, and detailed propagation analysis are critical for identifying concealed re-entrant circuits and achieving durable ablation success.
Frequently Asked Questions (FAQs)
1. What is atypical atrial flutter?
Atypical atrial flutter is a non-CTI–dependent macro-reentrant arrhythmia, often arising from the left atrium and commonly associated with scar tissue or prior ablation.
2. Why is atypical flutter harder to treat?
Because the circuit may involve complex scar-related pathways, making standard ECG interpretation and conventional mapping insufficient.
3. What role does scar tissue play?
Scar can both block conduction and create protected slow-conduction channels, which sustain re-entry.
4. Why adjust voltage thresholds during mapping?
Standard voltage cutoffs may label viable tissue as scar. Lowering thresholds reveals hidden conducting channels.
5. Is propagation mapping better than activation mapping?
Propagation mapping adds temporal visualization, making it easier to identify re-entrant pathways when activation maps are unclear.
6. What confirms successful ablation?
-
Termination of tachycardia during RF
-
Non-inducibility post-ablation
-
Stable sinus rhythm on ECG
7. Can atypical flutter recur?
Recurrence is possible, especially in progressive atrial disease, but targeted ablation significantly reduces risk.
8. Is this procedure safe?
When performed by experienced electrophysiologists, catheter ablation is a safe and effective treatment.